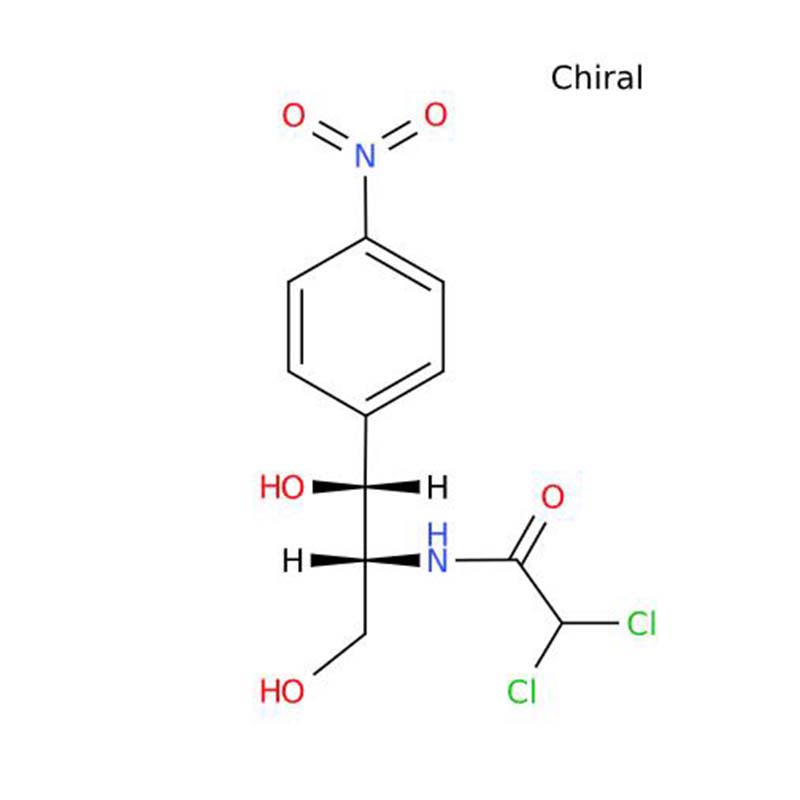
Chloramphenicol
Broad-Spectrum Antibiotic Activity: Chloramphenicol exhibits potent bacteriostatic effects against a diverse range of pathogens, demonstrating wide-ranging antibacterial utility.
Solubility and Stability Profile: It is readily soluble in methanol, ethanol, acetone, and ethyl acetate, and maintains stability in neutral or weakly acidic environments, facilitating flexible formulation development.
Isomeric Activity Differences: The naturally occurring L-isomer (levomycin) displays superior antibacterial efficacy, whereas synthetic preparations—typically racemic mixtures—possess approximately half the biological activity of the natural form.
Clinical Utility and Handling: Its broad-spectrum activity and chemical stability support clinical use in bacterial infection management, though optimal potency requires storage under dark, inert atmospheric conditions
Chloramphenicol (also termed levomycin), a broad-spectrum antibiotic derived from Streptomyces chlorinus, functions by inhibiting bacterial proliferation. The naturally occurring L-isomer constitutes the bioactive form, while synthetic chloramphenicol is typically obtained as white to faintly yellow needle-like or flaky crystals. It is odorless, exhibits a pronounced bitter taste, and demonstrates limited solubility in water, ether, and chloroform, though it dissolves readily in methanol, ethanol, acetone, and ethyl acetate. The compound is insoluble in benzene and petroleum ether.
This antibiotic remains stable in neutral or weakly acidic aqueous media but loses potency under alkaline conditions. Synthetic chloramphenicol is produced as a racemic mixture containing both bioactive L-isomer and inactive D-isomer. Since the D-form lacks antibacterial activity, the synthetic product exhibits only half the efficacy of natural chloramphenicol.
Parameters
Melting point | 148-150 °C(lit.) |
alpha | 19.5 º (c=6, EtOH) |
Boiling point | 644.9±55.0 °C(Predicted) |
density | 1.6682 (rough estimate) |
refractive index | 20 ° (C=5, EtOH) |
Fp | 14 °C |
storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
solubility | absolute ethanol: soluble5-20mg/mL (as a stock solution) |
form | |
pka | 11.03±0.46(Predicted) |
color | white |
Water Solubility | 2.5 g/L (25 º C) |
Merck | 142077 |
BRN | 2225532 |
BCS Class | 3 |
InChIKey | WIIZWVCIJKGZOK-RKDXNWHRSA-N |
LogP | 1.14 |
CAS DataBase Reference | 56-75-7(CAS DataBase Reference) |
NIST Chemistry Reference | Chloramphenicol(56-75-7) |
IARC | 2A (Vol. Sup 7, 50) 1990 |
EPA Substance Registry System | Chloramphenicol (56-75-7) |
Safety Information
Hazard Codes | T,F |
Risk Statements | 45-11-39/23/24/25-23/24/25 |
Safety Statements | 53-45-16-36/37 |
RIDADR | 2811 |
WGK Germany | 3 |
RTECS | AB6825000 |
F | 45726 |
TSCA | Yes |
HazardClass | 3 |
HazardClass | IRRITANT |
HS Code | 29414000 |
Hazardous Substances Data | 56-75-7(Hazardous Substances Data) |
Toxicity | LD50 oral in rat: 2500mg/kg |