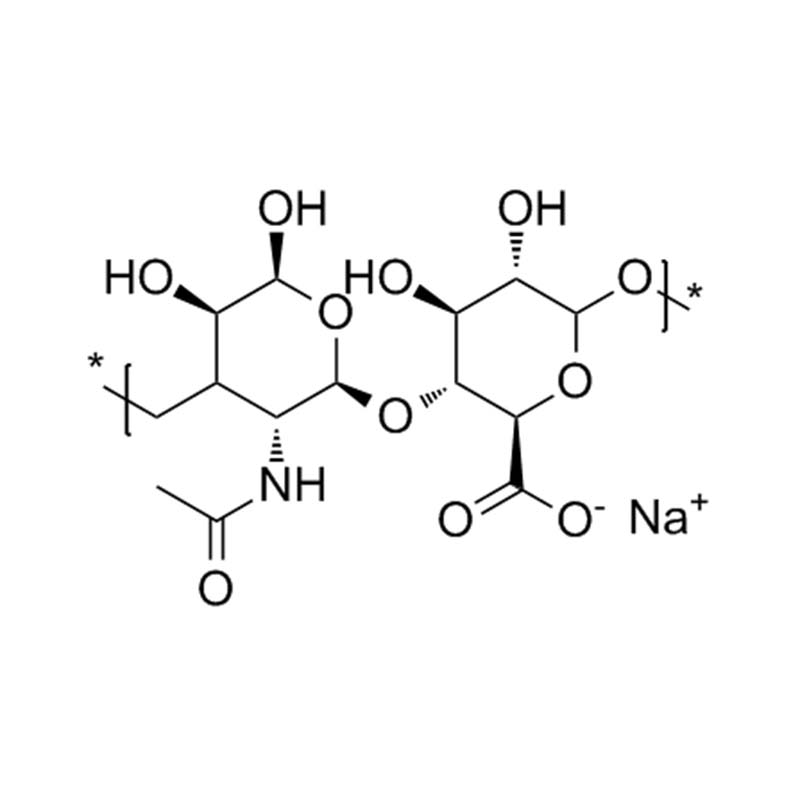
Sodium hyaluronate
Highly Biocompatible and Non-inflammatory– Sourced from physiological compounds naturally occurring in the human body, it minimizes inflammatory responses.
Superior Viscoelastic Properties – Delivers high viscosity and elasticity, making it well-suited for medical and surgical uses.
Ultra-High Purity and Sterility – With protein content under 0.5%, Healon meets stringent standards for purity and biocompatibility.
Fine-Gauge Injectability – Its flexible molecular structure enables smooth administration via very thin needles, enhancing ease of use in clinical settings.
Sodium hyaluronate, also known as sodium glycosaminoglycan, is a naturally occurring biological polymer widely present in human tissues. It is a high molecular weight linear polymer of the mucopolysaccharide type, built from repeating disaccharide units of glucuronic acid and acetylated hexosamine, with an average molecular weight of approximately one million. In aqueous solution, it forms a highly viscoelastic fluid that closely matches the pH and ionic environment of bodily fluids. Its flexible molecular configuration enables easy injection through fine-gauge needles. The purified, non-inflammatory form of sodium hyaluronate is commercially available under the trade name Healon. When dissolved in physiological saline at a concentration of 10 mg/mL, Healon exhibits a viscosity up to 200,000 times greater than that of aqueous humor or saline alone. It contains less than 0.5% protein, ensuring high purity and sterility suitable for medical applications.
Parameters
Melting point | >209°C (dec.) |
alpha | D25 -74° (c = 0.25 in water): Rapport et al., J. Am. Chem. Soc. 73, 2416 (1951) |
storage temp. | -20°C |
solubility | H2O: 5 mg/mL, clear, colorless |
form | |
color | White to cream |
PH | pH(2g/l,25℃) : 5.5~7.5 |
biological source | chicken (rooster comb) |
Water Solubility | SOLUBLE |
Merck | 13,4776 |
Stability: | Stable. Incompatible with strong oxidizing agents. |
InChI | InChI=1/C14H23NO11.Na/c1-4(17)15-5-2-6(18)7(3-16)24-14(5)26-10-8(19)9(20)13(23)25-11(10)12(21)22;/h5-11,13-14,16,18-20,23H,2-3H2,1H3,(H,15,17)(H,21,22);/q;+1/p-1/t5-,6+,7-,8-,9-,10+,11+,13-,14+;/s3 |
InChIKey | MAKUBRYLFHZREJ-JWBQXVCJSA-M |
SMILES | [C@@H]1(O[C@H]2[C@H](O)[C@H]([C@H](O)O[C@@H]2C(=O)[O-])O)O[C@H](CO)[C@@H](O)C[C@H]1NC(=O)C.[Na+] |&1:0,2,3,5,6,9,15,18,21,r| |
LogP | -6.623 (est) |
CAS DataBase Reference | 9067-32-7(CAS DataBase Reference) |
Safety Information
Hazard Codes | B,Xi |
Risk Statements | 36/37/38 |
Safety Statements | 22-24/25-36/37/39-27-26 |
WGK Germany | 3 |
RTECS | MT7250000 |
F | 3-10 |
HS Code | 39139000 |
Toxicity | LD50 oral in rabbit: > 1gm/kg |
Sodium hyaluronate is classified and applied across a wide spectrum of industries. It functions as a biochemical reagent—including both protein and carbohydrate categories—a certified reference material, and a foundational raw material in organic synthesis, cosmetics, pharmaceuticals, and nutraceuticals. It is utilized as a vitamin-enriched nutritional supplement, a food additive, a cosmetic hydrating agent, and an organic sodium salt. Additionally, it acts as a humectant in daily-use chemical products, an active pharmaceutical ingredient (API) or excipient in drug formulations, and a vital component in health supplements. Further applications include its role in enzyme and coenzyme preparations, impurity calibration standards, and select inorganic chemical uses.