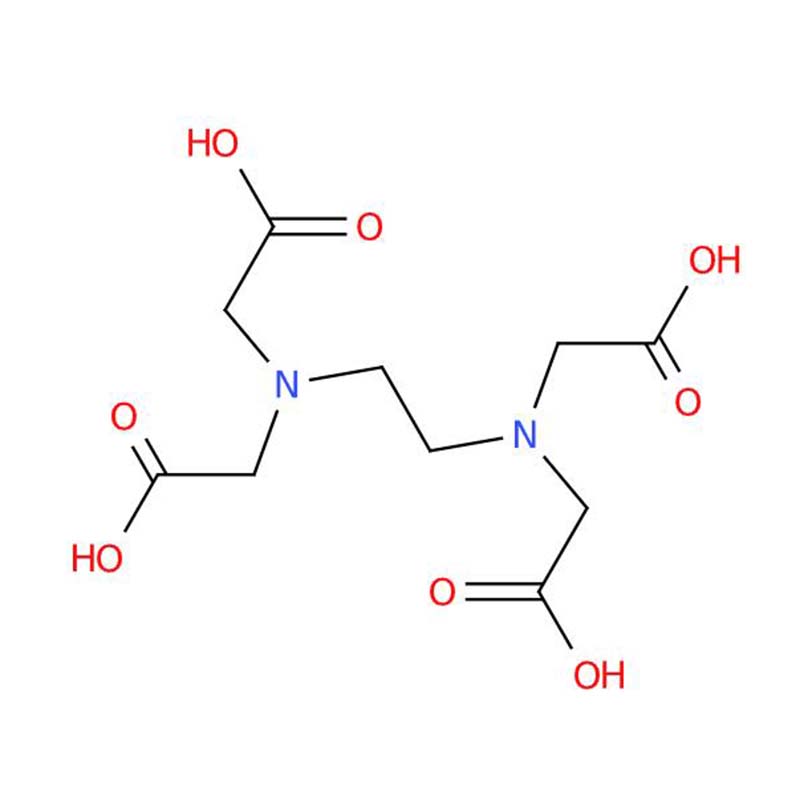
Ethylenediaminetetraacetic acid
Strong Chelating Ability: EDTA exhibits hexadentate chelation, forming highly stable coordination compounds through six-point binding with metal ions.
Effective Ligand: It functions as a potent polydentate ligand, enabling widespread use in industrial and laboratory settings for metal ion complexation.
Wide Application Range: This compound finds utility across diverse sectors including water treatment, pharmaceutical manufacturing, agricultural formulations, and as a specialty additive in chemical processes.
Chemical Stability: EDTA maintains structural integrity at elevated temperatures (up to 250°C) and demonstrates resistance to decomposition, ensuring reliability in demanding applications.
Ethylenediaminetetraacetic Acid (EDTA) functions as an extensively employed multidentate chelating agent. In aqueous media, EDTA undergoes complete deprotonation to generate the EDTA⁴⁻ anion. This anionic species exhibits six coordination sites: two nitrogen atoms and four oxygen atoms, which can simultaneously coordinate with a metal ion.
During complex formation with a metal ion, EDTA encapsulates the metal center via all six binding sites, producing a highly stable coordination complex. The secure coordination of EDTA to the metal ion is analogous to the grasping mechanism of a crustacean claw, elucidating the etymological origin of "chelation." The outstanding efficiency of EDTA as a chelating agent derives from its capability to engage with a metal ion at six distinct positions concurrently.
Ethylenediaminetetraacetic acid Chemical Properties
Melting point | 250 °C (dec.) (lit.) |
Boiling point | 434.18°C (rough estimate) |
bulk density | 600kg/m3 |
density | 1.46 g/cm3 at 20 °C |
vapor pressure | <0.013 hPa (20 °C) |
refractive index | n20/D 1.363 |
Fp | >400°C DIN 51758 |
storage temp. | 2-8°C |
solubility | 3 M NaOH: 100 mg/mL |
form | crystalline |
pka | pKa 2 (Uncertain);10.26 (Uncertain) |
color | White to almost white |
PH | 2.5 (10g/l, H2O, 23℃)(slurry) |
Odor | Odorless |
PH Range | 2.5 at 10 g/l at 23 °C |
Water Solubility | 0.5 g/L (25 ºC) |
λmax | λ: 280 nm Amax: ≤0.25 |
Decomposition | 240 °C |
Merck | 14,3517 |
BRN | 1716295 |
Stability: | Stable. Incompatible with copper, copper alloys, nickel, aluminium, strong oxidizing agents, strong bases |
LogP | -0.836 (est) |
CAS DataBase Reference | 60-00-4(CAS DataBase Reference) |
NIST Chemistry Reference | N,N'-1,2-Ethane diylbis-(N-(carboxymethyl)glycine)(60-00-4) |
EPA Substance Registry System | Ethylenediaminetetraacetic acid (60-00-4) |
Safety Information
Hazard Codes | Xi |
Risk Statements | 36-52/53-36/37/38-36/38 |
Safety Statements | 26-61-37/39-36 |
RIDADR | UN 3077 9 / PGIII |
WGK Germany | 2 |
RTECS | AH4025000 |
F | 3 |
Autoignition Temperature | >200 °C |
TSCA | Yes |
HS Code | 2922 49 85 |
Hazardous Substances Data | 60-00-4(Hazardous Substances Data) |
Toxicity | LD50 orally in Rabbit: 2580 mg/kg |