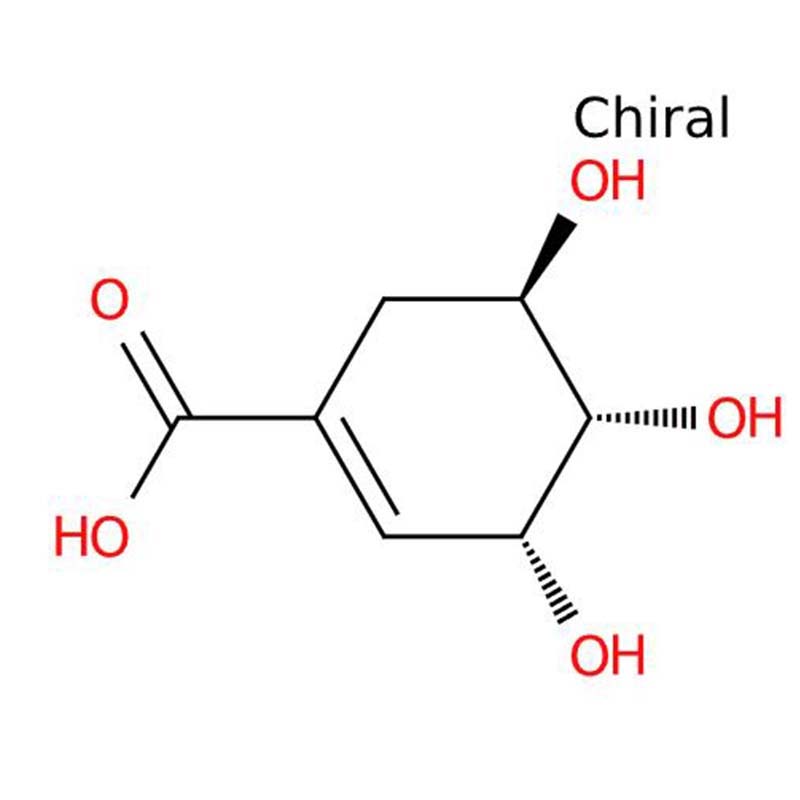
Shikimic acid
Pharmaceutical Intermediate: Serves as a crucial building block for synthesizing antiviral medications (including Tamiflu®) and antibiotic compounds.
High-Purity Production: Sourced through plant extraction from star anise (yielding 5-8%) or microbial fermentation (achieving 70 g/L titers) for industrial-scale manufacturing.
Biotechnological Applications: Incorporated into crop species for enhanced stress tolerance and employed in stereoselective synthesis processes.
Diverse Functional Applications: Functions as a preservative in food products, skin brightener in cosmetics, and anti-inflammatory component in therapeutics.
Sustainable Manufacturing: Advanced fermentation technologies substantially decrease dependence on botanical sources, dramatically reducing production costs by 80%.
Shikimic acid is a white crystalline cyclohexene carboxylic acid characterized by high water solubility (180 g/L at 20°C). The compound derives its name from Japanese shikimi grass (Illicium anisatum), from which it was first isolated. Functioning as a crucial metabolic intermediate in plants and microorganisms, it contains three chiral centers (3R,4S,5R configuration) and serves as the biochemical precursor for aromatic amino acids (phenylalanine, tyrosine, tryptophan) via the shikimate pathway.
While traditionally sourced from dried fruits of Illicium species, particularly Japanese star anise (Illicium anisatum), modern production has expanded to include extraction from sweetgum tree fruits (Liquidambar formosana) and pine needles. These alternative methods complement conventional sources and enhance supply sustainability for pharmaceutical and industrial applications.
Key Properties & Specifications
Parameter | Value |
Molecular Weight | 174.15 g/mol |
Melting Point | 185-187°C (decomp.) |
Specific Rotation | [α]₂₀ᴰ = -171° (c=1, H₂O) |
pKa Values | pKa₁=4.04, pKa₂=5.85 |
Log P (octanol-water) | -1.38 |
UV Absorption | λ<sub>max</sub> = 214 nm |
Thermal Stability | Degrades >200°C |
Production Methods & Sources
1. Traditional Plant Extraction
Source | Shikimic Acid Yield | Extraction Process |
Japanese Star Anise (Illicium anisatum) | 3-5% dry weight | - Acid hydrolysis (H₂SO₄) |
Chinese Star Anise (I. verum) | 5-8% dry weight | - Ethanol reflux |
Sweetgum (Liquidambar spp.) | 1.2-2.1% dry weight | - Alkaline hydrolysis |
Pine Needles (Pinus spp.) | 0.3-0.8% dry weight | - Supercritical CO₂ extraction |
2. Fermentation (Predominant Industrial Process)
Utilizing engineered E. coli strains (specifically K12/ΔaroL/ΔaroK) in glucose-based bioreactors, this method achieves high productivity, yielding titers exceeding 70 g/L. The fermentation is conducted under controlled conditions (37°C, pH 7.0) over a 60-hour period.
3. Chemical Synthesis
An asymmetric synthetic route employing a Diels-Alder reaction between furan and acrylic acid achieves high stereoselectivity (enantiomeric excess >98%). However, this method remains less economically viable, with production costs reaching $2,500/kg compared to $400/kg for fermentation-based production.
Industrial Applications
1. Pharmaceutical Synthesis
Serves as a critical precursor for antiviral agents including Oseltamivir Phosphate (Tamiflu®), meeting approximately 70% of global shikimic acid demand through a 10-step conversion process. Also utilized in producing Zanamivir (Relenza®), Peramivir, and antibiotics such as Chloramphenicol and mycobacterial inhibitors.
2. Biotechnology
Enables development of drought-resistant maize through shikimate pathway engineering with CRISPR technology. Functions as a chiral building block for synthesizing non-proteinogenic amino acids via biocatalytic processes.
3. Food & Agriculture
Acts as a plant elicitor to enhance phenolic compound synthesis in grapes, improving wine quality. Demonstrates antimicrobial efficacy against E. coli O157:H7 with a minimum inhibitory concentration of 12.5 mg/mL.
4. Cosmetics
Functions as a skin brightening agent through tyrosinase inhibition at 0.5-2% concentrations in serums. Provides anti-inflammatory benefits by suppressing TNF-α expression in keratinocytes.
Technical Grades & Specifications
Grade | Purity | Key Impurities | Applications |
Pharma Grade | >99.5% | Aromatic AA <0.1% | Antiviral synthesis |
Food Grade | >98.0% | Heavy metals <10 ppm | Functional ingredients |
Research Grade | >99.9% | Endotoxin <0.01 EU/mg | Enzyme kinetics studies |
Industrial | >95.0% | Water <0.5% | Agrochemical synthesis |
Packaging Options
Format | Capacity | Material |
Glass Vials | 1-100 g | Amber glass + PTFE seal |
HDPE Bottles | 0.5-5 kg | Nitrogen headspace |
Fiber Drums | 25 kg | Double PE liner |
Custom Bulk | >100 kg | Vacuum-sealed bags |