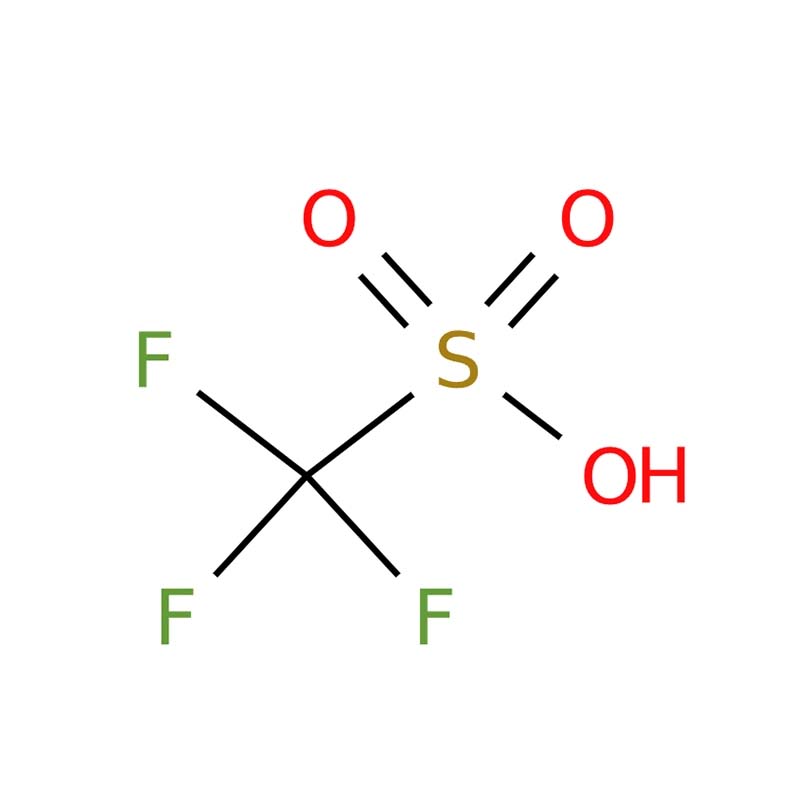
Trifluoromethanesulfonic acid
Superacid Strength: Triflic acid is classified as a superacid and ranks among the strongest acids known, which contributes to its high reactivity in various chemical reactions.
High Stability: The anhydrous form demonstrates high thermal and chemical stability, exhibiting strong resistance to both oxidation and reduction. This makes it particularly suitable for demanding fine chemical synthesis.
Versatile Applications: It serves as a key reagent and catalyst in the synthesis of diverse compounds, including pharmaceuticals, agrochemicals, and specialty polymers.
Non-Oxidizing Nature: Owing to its non-oxidizing properties, triflic acid is especially valuable in processes where oxidative side reactions must be avoided, such as in advanced pharmaceutical manufacturing.
Trifluoromethanesulfonic acid, also known as triflic acid (abbreviated as TFMS, TFSA, HOTf, or TfOH), is a powerful sulfonic acid with the molecular formula CF₃SO₃H. It is recognized as one of the strongest acids available and is categorized as a superacid. This compound is extensively utilized in the production of pharmaceuticals, agrochemicals, and polymeric materials. The anhydrous form is especially valued in fine chemical synthesis for its high stability and resistance to oxidation and reduction.
These characteristics, along with its non-oxidizing properties, establish triflic acid as a highly valuable reagent in diverse chemical processes. It plays an essential role in the pharmaceutical industry, particularly in synthesizing various drug classes such as nucleosides, antibiotics, steroids, proteins, and glycosides. Despite its broad utility, triflic anhydride—a common derivative—readily hydrolyzes upon contact with water and presents notable toxicity concerns, which restrict its use under many operational conditions.
Parameters
Melting point | -40 °C |
Boiling point | 162 °C (lit.) |
density | 1.696 g/mL at 25 °C (lit.) |
vapor density | 5.2 (vs air) |
vapor pressure | 8 mm Hg ( 25 °C) |
refractive index | n20/D 1.327(lit.) |
RTECS | PB2771000 |
Fp | None |
storage temp | Store below +30°C. |
solubility | Miscible in H<sub>2</sub>O |
pka | -14(at 25℃) |
form | Fuming Liquid |
color | slightly brown |
Specific Gravity | 1.696 |
PH | <1 (H2O) |
Water Solubility | SOLUBLE |
Sensitive | Hygroscopic |
Merck | 14,9676 |
BRN | 1812100 |
Stability | Stable. Incompatible with acids, alkalis, metals. |
InChIKey | ITMCEJHCFYSIIV-UHFFFAOYSA-N |
LogP | 0.3 at 25℃ |
CAS DataBase Reference | 1493-13-6(CAS DataBase Reference) |
NIST Chemistry Reference | CF3SO3H(1493-13-6) |
EPA Substance Registry System | Methanesulfonic acid, trifluoro- (1493-13-6) |
Safety Information
Hazard Codes | C |
Risk Statements | 21/22-35-10 |
Safety Statements | 26-36/37/39-45 |
RIDADR | UN 3265 8/PG 2 |
WGK Germany | 2 |
F | 3-10 |
Hazard Note | Corrosive/Hygroscopic |
TSCA | Yes |
HazardClass | 8 |
PackingGroup | II |
HS Code | 29049020 |
Toxicity | LD50 orally in Rabbit: 1605 mg/kg LD50 dermal Rat > 2000 mg/kg |